Here is a quick update on the ever-evolving world of COVID-19 trials. Since our initial posting on this topic three weeks ago, the number of trials has grown from 100 to over 300. The number of clinical facilities with clinical trials for COVID-19 has grown from 160 to over 600.
The fairly optimistic results about Gilead’s remdesivir, at the beginning of May, were a small sign of hope for patients and their families. You can seek entrance to these ongoing trials in over 140 facilities in the US and 70 in the EU (list of over 200 clinical facilities in the link below).
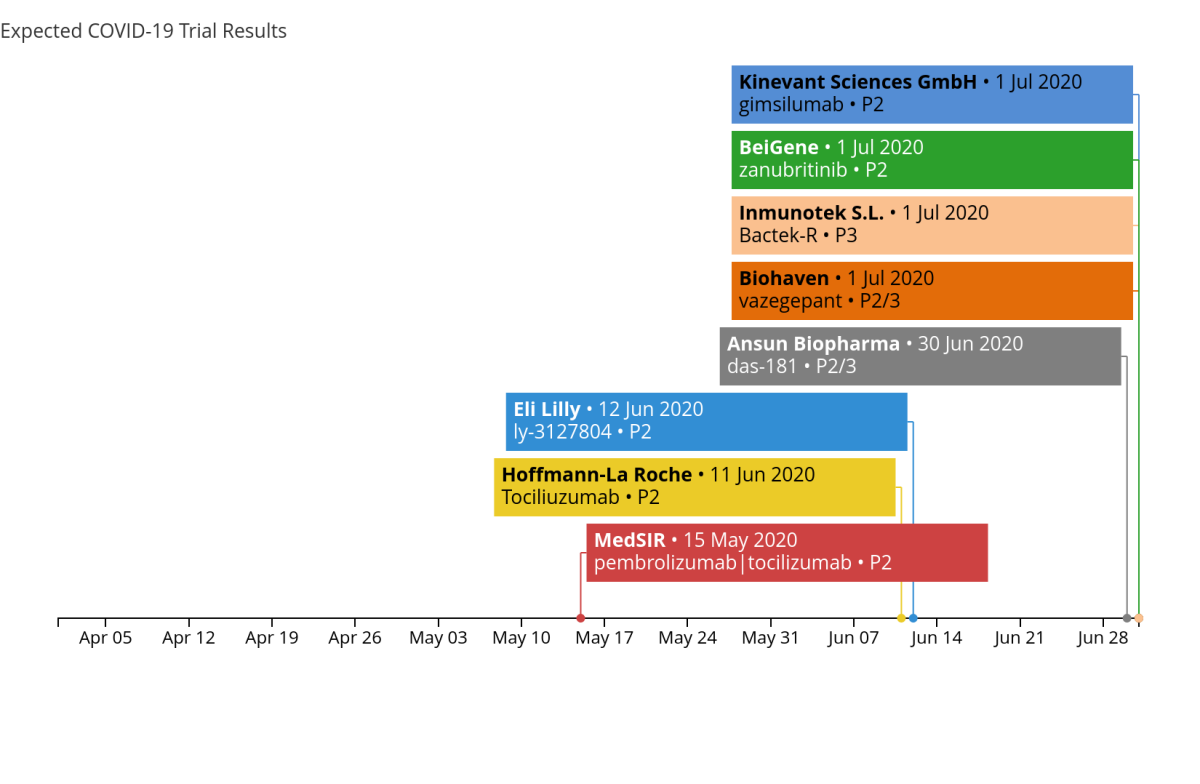
MedSir planned to have results for us in the next few days on the combination of pembrolizumab & tocilizumab in patients with mild acute respiratory syndrome. But based on the updates to their recruitment numbers, we are not certain those results will be on time.
Roche has recently entered the scene with a treatment of tocilizumab for patients with severe COVID-19, with results expected in the first couple of weeks in June. Following in the footsteps of Roche is Lilly’s ly-3127804, which accelerated their expected results from mid-July to mid-June.
Finally, there is a US-centered vaccine trial (healthy volunteers only) from Pfizer collaborating with BioNTech. The first two clinical locations are taking place at the University of Maryland and NYU Langone Health. This is an early trial to determine the safety and efficacy related to dosing for their RNA Vaccine. Stay tuned for more on that as they progress.
If you or someone you know are looking for treatment for COVID-19, please use our free list of hospitals and clinics that are currently treating patients for COVID-19. You can find this list by going to GlobalClinicalTrials. In addition to the list of facilities and their locations, we have added the treatment approach and a direct link to the clinical trial if you would like to inquire further about being a part of the trial.